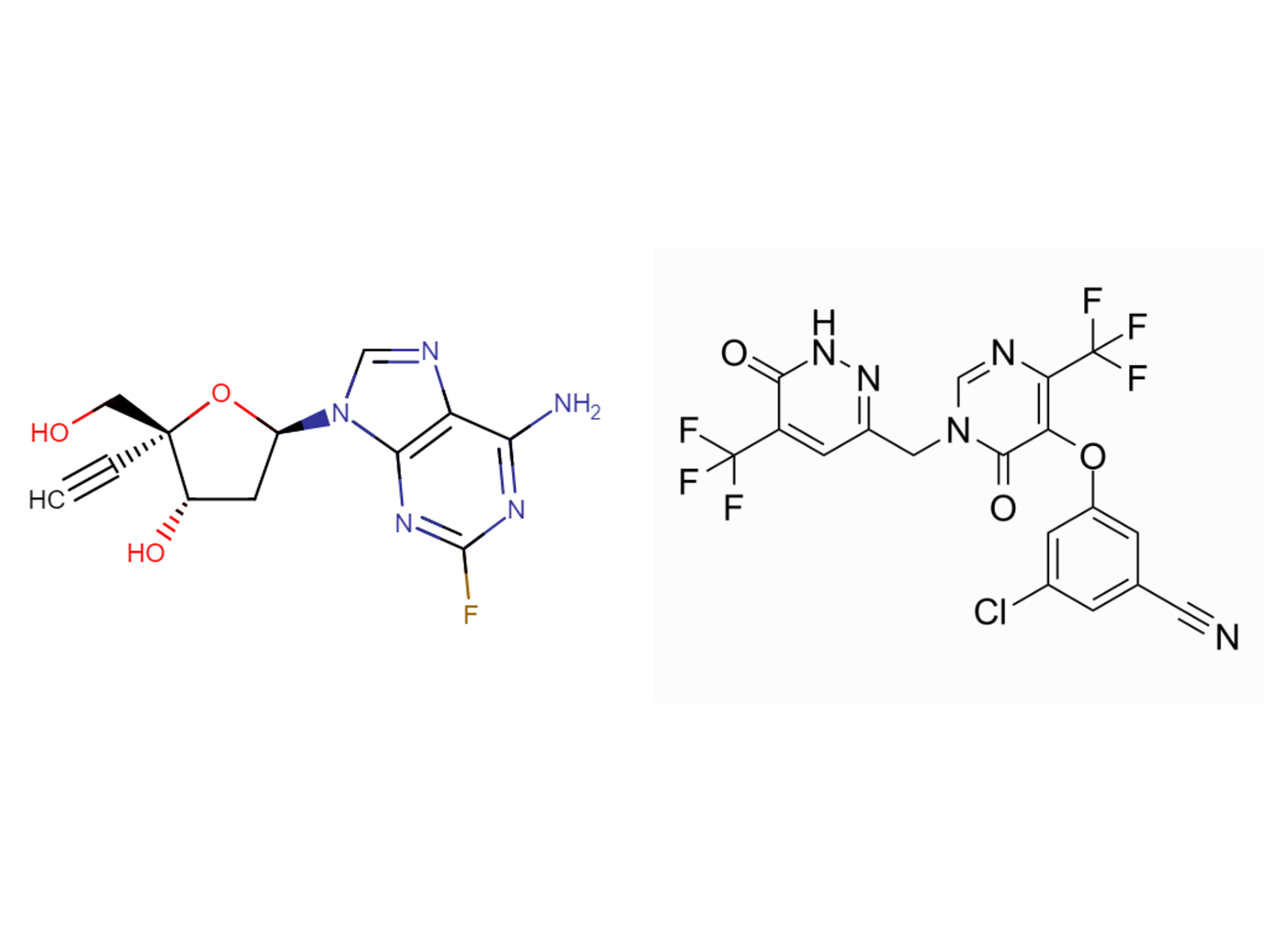
Drug information
Not provided
Not provided
Not provided
Small molecule
Not provided
Unknown
Unknown
Therapeutic area(s)
- HIV
- Treatment
Administration route
Oral
Associated long-acting platforms
Oral solid form
Use of drug
- Self-administered
Not provided
Dosage
Not provided
Not provided
Not provided
Not provided
Not provided
Not provided
Formulations
Compare
MK-8591B (islatravir + ulonivirine)
Associated technologies
Not provided
Scale-up and manufacturing prospects
Not provided
Not provided
Not provided
Not provided
Excipients
Not provided
Not provided
Not provided
Delivery device(s)
No delivery device
There are either no relevant patents or these were not yet submitted to LAPaL
Publications
Matthews RP, Patel M, Liu W, Liu Y, Rondón JC, Vargo RC, Stoch SA, Iwamoto M.2025.
Pharmacokinetics of islatravir in participants with moderate hepatic impairment.
Antimicrob Agents Chemother69:e01553-24.https://doi.org/10.1128/aac.01553-24
Islatravir (ISL) is a nucleoside reverse transcriptase translocation inhibitor in development for the treatment of HIV-1 infection. People living with HIV are at risk of liver disease. ISL is metabolized by adenosine deaminase (ADA), which is expressed in the liver; thus, ISL pharmacokinetics (PK) may be affected by hepatic impairment. This study evaluated the effect of moderate hepatic impairment on ISL PK. This nonrandomized, open-label, phase 1 study (MK-8591-030) evaluated the effects of a single oral dose of ISL 60 mg in HIV-seronegative adults with moderate hepatic insufficiency (n = 6) and matched healthy adult participants (n = 6). Blood samples for plasma ISL and 4′-ethynyl-2-fluoro-2′deoxyinosine (M4) and peripheral blood mononuclear cell (PBMC) ISL-triphosphate (ISL-TP) were collected at multiple time points through 672 h, and safety was monitored throughout. Modest decreases in maximum measured concentration (Cmax) and area under the concentration-time curve (AUC) of plasma ISL and AUC of PBMC ISL-TP were observed in participants with moderate hepatic impairment versus matched healthy participants, while ISL-TP Cmax was relatively unchanged. In contrast, plasma M4 was modestly increased in the moderate hepatic impairment group, suggesting that hepatic impairment may result in increased metabolism of ISL to M4 via ADA. The clinical relevance of the overall modest changes in M4, ISL, and ISL-TP levels with moderate hepatic impairment will be contextualized once exposure response data from ongoing clinical studies are available to elucidate the thresholds for clinical efficiency. A single oral dose of ISL 60 mg was generally well tolerated in both groups.
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing