Technology name
LYNX
Developer(s)
Sponsor(s)
Not specified
Type of technology
Oral solid form
Administration route
Oral
Development state and regulatory approval
Risperidone
Phase III
IND application was approved for LYN-005 by US FDA on 2020
Description
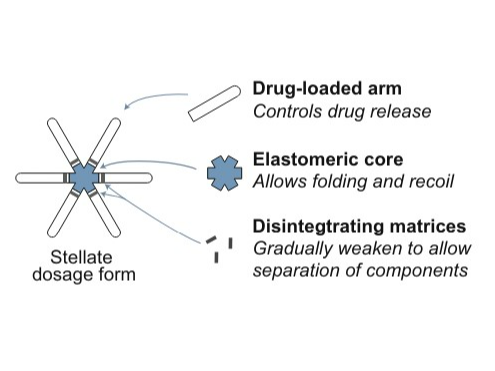
Lynx is an oral drug delivery system that releases the API slowly over time. It has the potential to decrease the number of times a drug needs to be taken from once a day to once a week. This system consists of a standard size capsule (00EL) with a core elastomer and six drug arms folded inside like a stellate. When the capsule dissolves, this stellate structure extends and lodges in the stomach for a week. It is gastric-resistant system which contains a carrier polymer component linked with one or more coupling polymers. This polymer is responsible for the extended release of the API.
Developer(s)

Lyndra Therapeutics
In 2015, Lyndra Therapeutics was founded by Robert Langer to create a pipeline of long-acting drugs. Since its creation, Lyndra has made significant progress, developing 25 medicines in the lab, finishing 12 clinical studies, and establishing a proof of concept for weekly oral dosing in 5 therapeutic areas, all of which validate the viability of its platform with various APIs.
Technology highlight
• LYNX consists of a central core attached with six polymer arms . • Each arm contains a concentrated amount of the API. • The capsule is coated with a proprietary material. • This coating makes it easy to swallow and ensures the capsule remains intact in the oesophagus, preventing premature drug release. • The elastomer material used in the arms of LYNX is porous. • This porosity allows for a slow and steady release of the drug into the system. • As a result, the therapeutic concentration of the API is maintained in the plasma for an extended period. • Thus, LYNX helps reduce the peaks and troughs in drug plasma levels. • The arms are connected to the core using biodegradable linkers. • Once the drug release is complete, these linkers soften and disintegrates.
Illustration(s)
Technology main components
(i) Carrier polymer (eg: polycaprolactone, polyanhydrides, polyphosphazenes, and polycyanoacrylates); (ii) API; (iii) Release enhancer; (iv) Dispersant (eg: carboxymethylcellulose, hypromellose, magnesium aluminum silicate, CABOSIL M-5P); (v) Solubilizer; (vi) Stabilizer (vii) Capsule coating (eg: Eudragit RS, Dichloromethane)
Not provided
Delivery device(s)
No delivery device
APIs compatibility profile
Not provided
Not provided
Not provided
50wt%
Not provided
Scale-up and manufacturing prospects
In terms of scale up prospective, Lyndra has begun ramping up new manufacturing operations in Lexington, Massachusetts. The plant began producing materials in April in preparation for the company's Phase II clinical trials, which are slated to begin later this year. In order to meet the demands of both upcoming and ongoing clinical studies as well as potential commercialization, Lyndra keeps growing its manufacturing capacity.
Haake MiniCTW, Twin-screw extruders, triangular cross-section rods, coating pan and dip coater.
• Initially, three 1-kg batches of a matrix formulation were produced and characterized for performance and stability. • Blends of drug, polymer, and excipients blended by continuous twin screw compounding at 500 g/h. • Blends are formed into triangular cross-section rods and cut to length to form drug arms. • Analysis showed good uniformity in both intra-batch and inter-batch. • The prepared formulation is dip-coated, assembled into stellate dosage forms, and analysed for storage stability.
HPLC with precolumn derivatization, NMR, X-ray diffraction and UV spectroscopy.
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Biodegradable
- Drug-eluting
- Room temperature storage
- At least 1 year shelf life
• The LYNX gastric residence system can release the API for a cumulative four to ten days, achieving near-zero-order drug release over a week. • The release characteristics are mainly based on the eudragit & dichloromethane-coated polymer matrix, which tends to release the API linearly over the first 24h once the surface drug is dissolved. • The dispersant added to the formulation also controls the initial burst release and maintains the percentage of drug release over a week. In addition to that, burst release and release rate can be modified by using varied concentrations of dispersants.
Not applicable
Interim analysis of ongoing clinical trials of LYN-005 (Oral Weekly Risperidone) shows positive results based on the PANSS score in schizophrenia. LYNN-005 is generally safe and well-tolerated.
LYNX gastric resistance system has an extended shelf life of three years
LYN-005 is meant to be stored at 15–25 °C. The capsules are to be handled carefully to avoid squeezing or crushing.
Therapeutic area(s)
- Malaria
- Contraception
- Other(s) : "Hyperlipidaemia and Pain Management"
- HIV
- Substance use disorders
- Mental health
- Treatment
Potential associated API(s)
- Risperidone
- rosuvastatin
- levomethadone
- Ivermectin
- memantine
- Dolutegravir (DTG)
- Rilpivirine (RPV)
- Cabotegravir (CAB)
- Opioids
- naloxone
Use of technology
- Self-administered
Weekly, Monthly
Not provided
Targeted user groups
- Adults
- Older Adults
- All
Unspecified
Unspecified
Yes
Not provided
Antipsychotic
Phase III
NCT04567524
Antipsychotic
Not provided
Once weekly
IND application was approved for LYN-005 by US FDA on 2020
rosuvastatin
HMG-CoA reductase inhibitor
Phase I
ACTRN12621000101886
Hyperlipidemia
Not provided
Once weekly
Not provided
levomethadone
Drug Abuse
Phase I
NCT05251376
Opioid Use Disorder
Not provided
Once weekly
Received IND on May 2021 and Fast Track designation from FDA
Antimalarial
Phase I
ACTRN12621001218886
Malaria infection
Not provided
Once every two weeks
Not provided
memantine
NMDAR antagonist
Pre-clinical
Not provided
Alzheimer's disease
Not provided
Once weekly
Not provided
Dolutegravir (DTG)
HIV integrase inhibitor
Pre-clinical
Not provided
HIV
Not provided
Once a week
Not provided
Rilpivirine (RPV)
Non-nucleoside reverse transcriptase inhibitors
Pre-clinical
Not provided
HIV
Not provided
Once a week
Not provided
Cabotegravir (CAB)
HIV integrase inhibitors
Pre-clinical
Not provided
HIV
Not provided
Once a week
Not provided
Opioids
Narcotic analgesic
Pre-clinical
Not provided
Pain Management
Not provided
Once a week
Not provided
naloxone
Drug Abuse
Pre-clinical
Not provided
Opioid dependence
Not provided
Once a week
Not provided
Gastric residence systems with release rate-modulating films
he invention provides gastric residence systems, or components of gastric residence system such as segments or elongate members of gastric residence systems, with release rate- modulating films and methods for making and using such systems. The release rate-modulating films provide good control over release of agents (such as therapeutic, diagnostic, or nutritional agents) present in the gastric residence system. The films also permit higher drug loading in the gastric residence systems and components of gastric residence systems while maintaining good control over release of agents. Some embodiments of the films can provide resistance against burst release of agent upon exposure to alcohol.
WO2018227147
Device
Lyndra Therapeutics
Not provided
June 8, 2038
Granted: AU, JP, US Pending: CA, EP, CN
Gastric residence systems for sustained release of therapeutics agents and methods of use thereof
Gastric residence systems comprise therapeutic agent formulations for sustained gastric release of therapeutic agents as well as methods for using such systems. The systems are by using a dispersant in the formulations, which improves the burst release characteristics and long-term release rate characteristics of the systems. Milling of the therapeutic agent can be performed to prepare agent particles of the desired size.
WO2017070612
Device
Lyndra Therapeutics
Not provided
October 21, 2036
Granted: AU, CA, JP, US Pending: EP, CN
A multi-armed star shaped gastric residence structure loaded with therapeutic agent
Residence structures, systems, and related methods are generally provided. Certain embodiments comprise administering (e.g., orally) a residence structure to a subject (e.g., a patient) such that the residence structure is retained at a location internal to the subject for a particular amount of time (e.g., at least about 24 hours) before being released. The residence structure may be, in some cases, a gastric residence structure. In some embodiments, the structures and systems described herein comprise one or more materials configured for high levels of active substances (e.g., a therapeutic agent) loading, high active substance and/or structure stability in acidic environments, mechanical flexibility and strength in an internal orifice (e.g., gastric cavity), easy passage through the GI.
WO2015191920
Device
MIT; Brigham & Women's Hospital; Tokitae LLC
patents licensed exclusively to LYNDRA INC. from Massachusetts Institute of Technology and Brigham & Women’s Hospital
June 11, 2035
Granted: AU, BR, CA, CN, EP (BE, CH, DE, FR, GB, LI, LU), HK, IL, JP, KR, MX, NZ, RU, SG, US, ZA
Publications
Kanasty, R., Low, S., Bhise, N., Yang, J., Peeke, E., Schwarz, M., ... & Bellinger, A. M. (2019). A pharmaceutical answer to nonadherence: Once weekly oral memantine for Alzheimer's disease. Journal of controlled release, 303, 34-41. https://doi.org/10.1016/j.jconrel.2019.03.022
The formulation of memantine hydrochloride is the first oral dosage form to achieve sustained drug release for a week with near zero-order kinetics and efficient delivery. In the dog model, relative memantine bioavailability approaches 100%, with sustained plasma levels over seven days. A single gastric resistant dosage form achieves an AUC equivalent to seven daily treatments with the marketed daily capsule, with a Cmax no higher than the daily product. This formulation methodology is applicable to many water-soluble drugs and may enable the development of long-acting oral therapies for various conditions.
Kirtane AR, Abouzid O, Minahan D, Bensel T, Hill AL, Selinger C, Bershteyn A, Craig M, Mo SS, Mazdiyasni H, Cleveland C, Rogner J, Lee YL, Booth L, Javid F, Wu SJ, Grant T, Bellinger AM, Nikolic B, Hayward A, Wood L, Eckhoff PA, Nowak MA, Langer R, Traverso G. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat Commun. 2018 Jan 9;9(1):2. doi: 10.1038/s41467-017-02294-6.
The efficacy of antiretroviral therapy is significantly compromised by medication non-adherence. Long-acting enteral systems that can ease the burden of daily adherence have not yet been developed. Here we describe an oral dosage form composed of distinct drug-polymer matrices which achieved week-long systemic drug levels of the antiretrovirals dolutegravir, rilpivirine and cabotegravir in a pig. Simulations of viral dynamics and patient adherence patterns indicate that such systems would significantly reduce therapeutic failures and epidemiological modelling suggests that using such an intervention prophylactically could avert hundreds of thousands of new HIV cases. In sum, weekly administration of long-acting antiretrovirals via a novel oral dosage form is a promising intervention to help control the HIV epidemic worldwide.
Foltin, R. W., Zale, S., Sykes, K. A., Nagaraj, N., Scranton, R. E., & Comer, S. D. (2022). A novel long-acting formulation of oral buprenorphine/naloxone produces prolonged decreases in fentanyl self-administration by rhesus monkeys. Drug and alcohol dependence, 239, 109599. https://doi.org/10.1016/j.drugalcdep.2022.109599
We evaluated the efficacy of this formulation in reducing intravenous (i.v.) fentanyl self-administration by three male and three female rhesus monkeys. Buprenorphine HCl and naloxone HCl were co-formulated using an 11:1 ratio of buprenorphine:naloxone in a controlled-release gastric residence formulation administered in an oral capsule (LYN-013). Naloxone was included to determine the feasibility of combining naloxone with buprenorphine in the formulation as an abuse deterrent. Complete fentanyl dose-response functions were determined during each session. The efficacy of single doses of 56/5, 112/10 and 168/15 mg buprenorphine/naloxone in reducing fentanyl self-administration was examined over 13 days. LYN-013 significantly decreased the rate of responding for fentanyl for 3 days and significantly reduced total intake of fentanyl for 8 days. Time to maximal buprenorphine levels (Tmax) ranged between 56 and 68 h for all 3 doses. The maximal buprenorphine level (Cmax) following 168 mg was 2.3 ng/ml which was significantly greater that those observed for 56 mg (1.22 ng/ml) and 112 mg (1.35 ng/ml). Finally, the area-under-curves (AUCtau) were buprenorphine dose-dependently increased from 88 to 127-265 h*ng/ml. There were no signs of non-specific changes in behavior.
Additional documents
No documents were uploaded
Useful links
There are no additional links
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
All sponsors
No sponsor indicated



