Technology name
Refillable Nanofluidic Implant
Developer(s)
Sponsor(s)
Type of technology
Titanium implant
Administration route
Transdermal
Development state and regulatory approval
Islatravir (ISL)
Pre-clinical
Not provided
Description
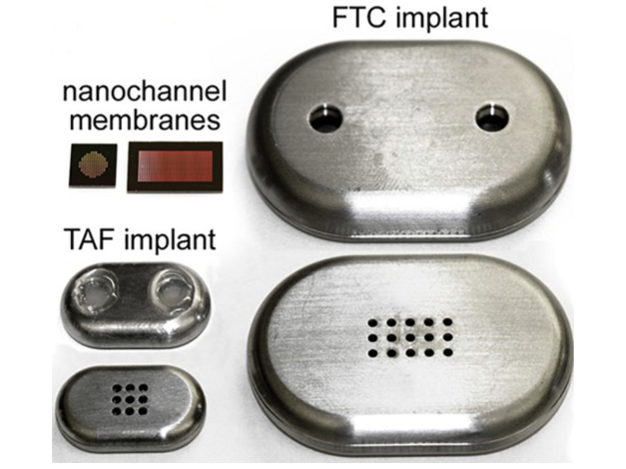
Refillable nanofluidic implants have been designed as an innovative solution for the long-acting delivery of pre-exposure prophylaxis (PrEP) against HIV. Each implant is composed of a titanium reservoir with a volume capacity of 570 μl, integrated with two self-sealing silicone ports to facilitate drug loading and refilling processes. The nanofluidic design incorporates a titanium structure coated to enable the controlled elution of the API over a duration of up to four months. Notably, the refillable nature of these implants supports the efficient loading of solid drug particles.
Developer(s)

Houston Methodist Research Institute
Houston Methodist Research Institute (HMRI), founded in 2004, is the academic arm of Houston Methodist Hospital in Texas. It focuses on translational research, bridging lab discoveries to clinical applications. HMRI specializes in areas like regenerative medicine, nanomedicine, oncology, and artificial intelligence in healthcare. Its state-of-the-art facilities include a dedicated nanomedicine lab
Technology highlight
1) The API exhibits zero-order kinetics, ensuring a constant release rate, even at low dose concentrations. 2) Steady-state drug levels are achieved within 48 hours of administration. 3) The size of the drug microparticles does not influence the release kinetics, demonstrating robust and uniform performance. 4) The steady-state concentration is maintained for up to 20 months, ensuring prolonged efficacy.
Illustration(s)
Technology main components
1) Titanium-polymeric shell 2) Biocompatible carbide-coated silicon membrane with 278,600 nanochannels within an 8-mm2 surface area 3) API Reservoir 4) Loading/refill ports
The National Institute of Allergy and Infectious Diseases (NIAID), USA, provided support for all raw materials utilized during initial phase of development. Price is not disclosed
Delivery device(s)
No delivery device
APIs compatibility profile
Antiretrovirals such as Islatravir, Tenofovir, Efavirenz, Lamivudine, Emtricitabine, Dolutegravir, Raltegravir, Bictegravir, Maraviroc, Atazanavir, and Darunavir are targeted for Refillable nanofluidic implants.
Not provided
Not provided
75-90 wt%
1 single API :
Min: -1.3
Max: 4.7
Islatravir LogP - 0.7
Scale-up and manufacturing prospects
Not provided
Not provided
The manufacturing process includes: 1) Nanofluidic membrane: Silicon nanofluidic membranes were fabricated on silicon-on-insulator wafers using semiconductor techniques. 2) Titanium shell: These membranes were mounted onto medical-grade 6AI4V titanium drug reservoirs using UV epoxy. 3) Assembly: A titanium filter was secured between the reservoir shells with silicone O-rings. The implants were arc-welded, primed in 1x PBS under vacuum, and gamma-sterilized at 30kGy.
1. Optical imaging (EVOS microscope) 2. Scanning electron microscopy (SEM) 3. HPLC
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Additional features
- Drug-eluting
- Removable
- Refillable
- Reusable
- Reservoir-type
- Room temperature storage
- At least 1 year shelf life
- Needs insertion kit
• Triphasic release kinetics • Zero-order kinetics (unaffected by the size of the drug microparticle)
• The API liquid is introduced into the reservoir chamber using 19- and 25-gauge needles. • The implantation procedure necessitates general anesthesia and a 1-cm incision.
• In a 20-month nonhuman primate study, islatravir-eluting nanofluidic implants demonstrated excellent tolerability, with mild local tissue inflammation and no systemic toxicity observed. • Moreover, the implants provided 100% protection against SHIV infection.
• Stability studies, as assessed by HPLC chromatography, demonstrated the integrity of the API within the implants over a 9-month period.
Not provided
Therapeutic area(s)
- HIV
- Pre-Exposure Prophylaxis (PrEP)
Potential associated API(s)
Use of technology
- Administered by a community health worker
- Administered by a nurse
- Administered by a specialty health worker
Every 6 months
Not provided
Targeted user groups
- Adults
- Older Adults
- All
Unspecified
Unspecified
Yes
Not provided
Nucleoside and nucleotide reverse transcriptase inhibitors (NRTI)
Pre-clinical
Not provided
HIV and AIDS
Not provided
2 years
Not provided
HIV-1 capsid protein shell inhibitors
Pre-clinical
Not provided
HIV
Not provided
2 years
Not provided
Nucleoside and nucleotide transcriptase inhibitors
Pre-clinical
Not provided
HIV
Not provided
2 years
Not provided
There are either no relevant patents or these were not yet submitted to LAPaL
Publications
Chua CYX, Jain P, Ballerini A, et al. Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis. J Control Release. 2018;286:315-325. doi:10.1016/j.jconrel.2018.08.010
Pre-exposure prophylaxis (PrEP) with antiretroviral (ARV) drugs are effective at preventing human immunodeficiency virus (HIV) transmission. However, implementation of PrEP presents significant challenges due to poor user adherence, low accessibility to ARVs and multiple routes of HIV exposure. To address these challenges, we developed the nanochannel delivery implant (NDI), a subcutaneously implantable device for sustained and constant delivery of tenofovir alafenamide (TAF) and emtricitabine (FTC) for HIV PrEP. Unlike existing drug delivery platforms with finite depots, the NDI incorporates ports allowing for transcutaneous refilling upon drug exhaustion. NDI-mediated drug delivery in rhesus macaques resulted in sustained release of both TAF and FTC for 83 days, as indicated by concentrations of TAF, FTC and their respectively metabolites in plasma, PBMCs, rectal mononuclear cells and tissues associated with HIV transmission. Notably, clinically relevant preventative levels of tenofovir diphosphate were achieved as early as 3 days after NDI implantation. We also demonstrated the feasibility of transcutaneous drug refilling to extend the duration of PrEP drug delivery in NHPs. Overall, the NDI represents an innovative strategy for long-term HIV PrEP administration in both developed and developing countries.
Pons-Faudoa FP, Di Trani N, Capuani S, et al. Long-acting refillable nanofluidic implant confers protection against SHIV infection in nonhuman primates. Sci Transl Med. 2023;15(702):eadg2887. doi:10.1126/scitranslmed.adg2887
The impact of pre-exposure prophylaxis (PrEP) on slowing the global HIV epidemic hinges on effective drugs and delivery platforms. Oral drug regimens are the pillar of HIV PrEP, but variable adherence has spurred development of long-acting delivery systems with the aim of increasing PrEP access, uptake, and persistence. We have developed a long-acting subcutaneous nanofluidic implant that can be refilled transcutaneously for sustained release of the HIV drug islatravir, a nucleoside reverse transcriptase translocation inhibitor that is used for HIV PrEP. In rhesus macaques, the islatravir-eluting implants achieved constant concentrations of islatravir in plasma (median 3.14 nM) and islatravir triphosphate in peripheral blood mononuclear cells (median 0.16 picomole per 106 cells) for more than 20 months. These drug concentrations were above the established PrEP protection threshold. In two unblinded, placebo-controlled studies, islatravir-eluting implants conferred 100% protection against infection with SHIVSF162P3 after repeated low-dose rectal or vaginal challenge in male or female rhesus macaques, respectively, compared to placebo control groups. The islatravir-eluting implants were well tolerated with mild local tissue inflammation and no signs of systemic toxicity over the 20-month study period. This refillable islatravir-eluting implant has potential as a long-acting drug delivery system for HIV PrEP.
Di Trani N, Pons-Faudoa FP, Sizovs A, et al. Extending drug release from implants via transcutaneous refilling with solid therapeutics. Adv Ther (Weinh). 2022;5(2):2100214. doi:10.1002/adtp.202100214
Long-acting (LA) implantable drug delivery systems (IDDS) offer an effective approach for the management or prevention of chronic conditions by sustained parenteral therapeutic administration. LA IDDS can and improve adherence to treatment regimens by minimizing dosing frequency. However, their clinical deployment is challenged by factors such as poor drug loading capacity, which limit their lifespan and require repeated surgical replacement for continued therapy. To address these challenges, and by leveraging previous work on nanofluidic systems, a reservoir-based IDDS that enables transcutaneous refilling of solid drug formulations through minimally invasive needle injection is presented. With thousand-fold higher drug loading efficiency, the implant affords minimal volume and aspect ratio suitable for discrete subcutaneous deployment. Key parameters for clinical acceptability, namely implant safety, access port robustness, and refilling method were systematically evaluated. The implant and refilling procedure are studied in rats and nonhuman primates with therapeutics used clinically for type 2 diabetes and human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP). The ability to extend drug release and maintain equivalent pharmacokinetics (PK) profiles pre- and post-drug refilling is demonstrated. This technology presents a clinically viable LA approach to prolong drug release for lifelong prevention or management of chronic conditions.
Additional documents
No documents were uploaded
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing
