Drug information
Not provided
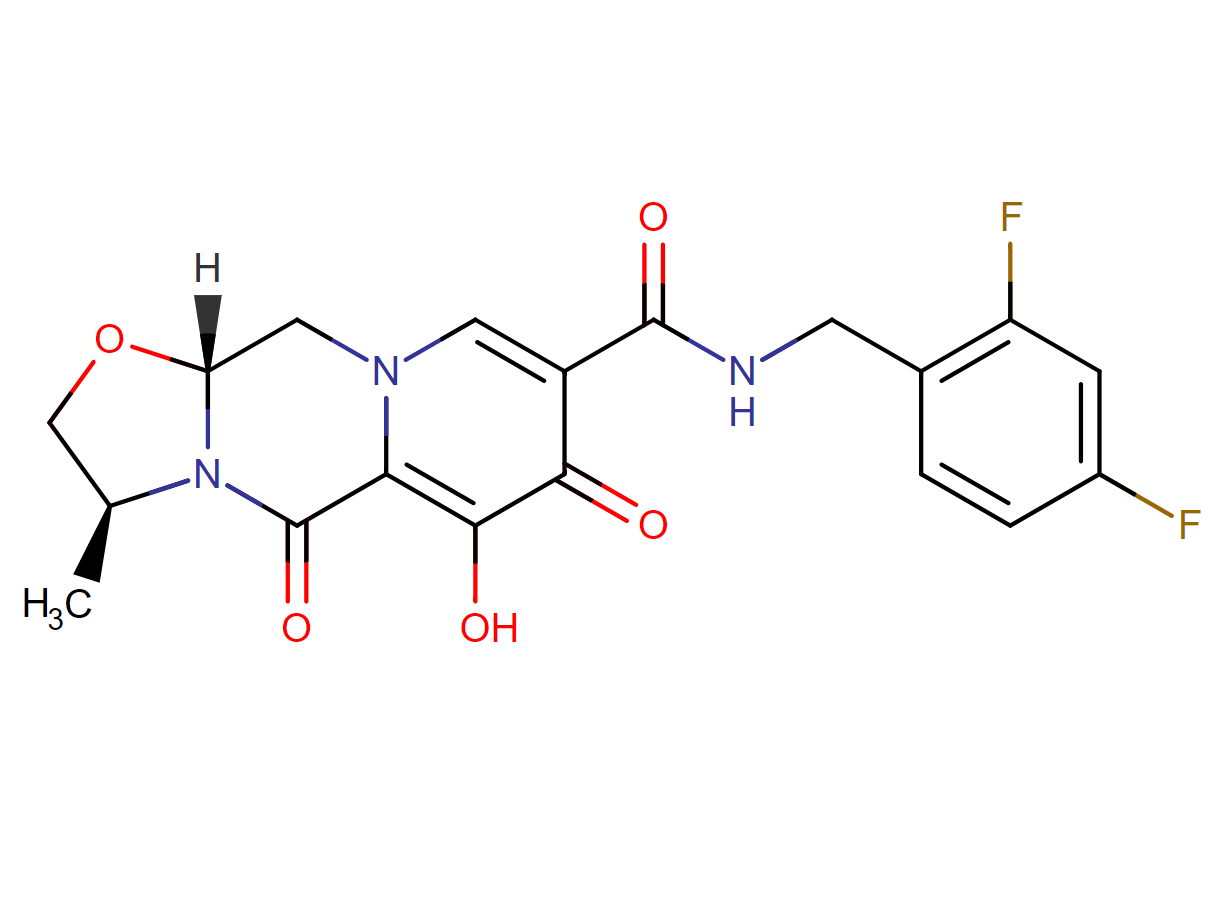
Cabotegravir
Apretude, Vocabria
Small molecule
Cabotegravir (CAB), also known as GSK1265744, is a HIV-1 integrase strand transfer inhibitor (INSTI) used for pre-exposure prophylaxis (PrEP) and the treatment of individuals infected with HIV. CAB is utilised in combination with Rilpivirine (a non-nucleoside reverse transcriptase inhibitor, NNRTI) for HIV treatment. Long-acting versions of CAB (CAB-LA) are currently administered once monthly or every-2-months as a intramuscular (IM) injection containing an extended-release drug particle nanosuspension, with an optional ~30 day oral-lead in period. CAB-LA administered by IM injection requires approximately one week to achieve maximal plasma drug concentration. CAB is metabolised in the body by the enzyme UGT1A1; with the average half-life of IM CAB-LA ranging from 5.6 to 11.5 weeks.
CAB-LA (APRETUDE) 600mg/3mL extended-release IM injectable suspension single dose vials have received approval for use in HIV-1 PrEP for HIV-negative adults and adolescents weighing ≥ 35 kilograms who are at risk of infection. CAB-LA (VOCABRIA) is used together with another medicine called rilpivirine (REKAMBYS) (or co-packed as a2-drug co-packaged product CABENUVA) as a complete regimen for the treatment to treat adults (and children, depending on approvals) living with human HIV-1. VOCABRIA is available as tablets to be taken by mouth and as a prolonged-release suspension for injection.
CAB-LA has been designated as a Breakthrough Therapy by the USFDA, granted Extension of Indications approval by TGA Australia, and awarded European Marketing Authorization by the EMA. It is indicated for individuals without prior HIV-1 infection and devoid of any indications of drug resistance. Medicine Control Authority of Zimbabwe was the first African regulatory body to approve CAB-LA for HIV-PrEP. Furthermore, the World Health Organization's Guideline Development Group has conditionally recommended CAB-LA as an adjunctive preventive measure for those at substantial risk of HIV-1 infection.
Therapeutic area(s)
- HIV
- Pre-Exposure Prophylaxis (PrEP)
- Treatment
Administration route
Oral, Intramuscular
Associated long-acting platforms
Aqueous drug particle suspension
Use of drug
- Administered by a nurse
- Administered by a specialty health worker
Not provided
Dosage
600 mg/3 mL
Once monthly (Q1M); Two months once (Q2M)
200 mg/mL
Not provided
Not provided
Formulations
Compare
Cabotegravir 4-monthly (Q4M)
Cabotegravir Stearate (M2CAB)
Cabotegravir and Rilpivirine
cabotegravir PH20
Associated technologies
Dissolving microarray patchesTunable Biodegradable Ultra-Long-Acting Polymeric Solid Implant (PSI)
Ultra-Long-Acting Multi-Purpose In-situ Forming Implant (ISFI)
DelSiTech Silica Matrix Drug Delivery
Peptide-like hydrogels as a long-acting injectable drug delivery platform
LYNX
ProTide Prodrug Technology
Comment & Information
Developer(s)

ViiV Healthcare is a pharmaceutical company that specializes in the development of therapies for HIV infection. The company is headquartered in Brentford in the United Kingdom and was initially formed in November 2009 as a part of a joint venture between GlaxoSmithKline and Pfizer.
Drug structure
Scale-up and manufacturing prospects
Compound is commercially manufactured by the innovator and three generic manufacturers have received a licence through the medicines patent pool to manufacture generic versions by 2026/2027.
Conventional wet-bead milling (ball mill), depyrogenated glass vials.
Cabotegravir is subject to a gamma-irradiation pre-sterilization step prior to a conventional wet-bead milling manufacturing procedure. The Cabotegravir milling process is initiated alongside pharmaceutical excipients (polyethylene glycol 3350, water for injection, polysorbate 20 and mannitol) for an overall 200nm drug particle size. Sterilized de-pyrogenated glass vials are used to store the finished drug nanosuspension, before an additional gamma irradiation (25kGy) step to ensure aseptic packaging conditions.
PANalytical X’Pert PRO diffractometer equipped with a theta/theta coupled goniometer (or equivalent x-ray powder diffractor) to determine drug particle size, Mettler TGA/DSC 1 instrument for thermal analysis, HPLC to evaluate drug content, impurities and dissolution, HPLC UV-Vis Detector for drug identification.
Excipients
No proprietary excipient used
No novel excipient or existing excipient used
No residual solvent used
Delivery device(s)
No delivery device
Publications
Bowers GD, Culp A, Reese MJ, Tabolt G, Moss L, Piscitelli S, Huynh P, Wagner D, Ford SL, Gould EP, Pan R, Lou Y, Margolis DA, Spreen WR: Disposition and metabolism of cabotegravir: a comparison of biotransformation and excretion between different species and routes of administration in humans. Xenobiotica. 2016;46(2):147-62. doi: https://doi.org/10.3109/00498254.2015.1060372 Epub 2015 Jul 1
1. Cabotegravir [(3S,11aR)-N-[(2,4-difluorophenyl)methyl]-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide] is an HIV-1 integrase inhibitor under development as a tablet for both oral lead-in therapy and long-acting (LA) injectable for intramuscular dosing.
2. Metabolism, pharmacokinetics and excretion were investigated in healthy human subjects who received either a single oral dose (28.2 mg) of [14C]cabotegravir in a mass balance study, or LA formulations of unlabeled cabotegravir (200–800 mg), intramuscularly or subcutaneously, in a separate study. Metabolism, distribution and excretion of [14C]cabotegravir were also investigated in mice, rats and monkeys.
3. Recovery of radioactivity in humans represented a mean total of 85.3% of the dose, including 26.8% in the urine. The mean apparent terminal phase half-life was similar for both cabotegravir and radioactivity, 39 h compared to 41 h.
4. Following oral, intramuscular and subcutaneous administration, cabotegravir was the major component in plasma and the glucuronic acid conjugate (M1) represented the predominant component in urine. Cabotegravir was present in bile along with its major metabolite (M1).
5. The primary metabolite of [14C]cabotegravir in mouse, rat and monkey was the same as that in human. In vitro phenotyping experiments demonstrated that cabotegravir was metabolized by UDP-glucuronosyltransferase (UGT) 1A1 and UGT1A9.
Spreen W, Min S, Ford SL, Chen S, Lou Y, Bomar M, St Clair M, Piscitelli S, Fujiwara T: Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013 Sep-Oct;14(5):192-203. doi: https://doi.org/10.1310/hct1405-192
Background: GSK1265744 is an HIV integrase strand transfer inhibitor selected for clinical development.
Objective: This first-time-in-human and phase IIa investigation assessed GSK1265744 antiviral activity, pharmacokinetics, safety, and tolerability in healthy and HIV-1-infected subjects.
Methods: This double-blind, placebo-controlled study consisted of a dose escalation of single (part A) and multiple (part B) oral doses in 48 healthy subjects and an oral dose (part C) in 11 HIV-1-infected subjects. In part A, 2 cohorts of 9 subjects received either 5 and 25 mg or 10 and 50 mg. In part B, 3 cohorts of 10 subjects received 5, 10, or 25 mg once daily for 14 days. In part C and the phase IIa study, subjects received 5 or 30 mg once daily for 10 days.
Results: Dose-proportional increases in drug exposure were observed in healthy and HIV-1-infected subjects. In healthy subjects, pharmacokinetic variability was low following single or repeat dosing (coefficient of variation, 13%-34% and 15%-23%, respectively). Mean plasma half-life was 31.5 hours. GSK1265744 monotherapy significantly reduced plasma HIV-1 RNA from baseline to day 11 in HIV-1-infected subjects receiving 5 or 30 mg versus placebo (P < .001); mean decrease was 2.2 to 2.3 log10 copies/mL, respectively. Study drug was generally well tolerated with no clinically relevant trends in laboratory values, vital signs, or electrocardiograms.
Conclusions: GSK1265744 was well tolerated in healthy and HIV-1-infected subjects. Results demonstrate once-daily doses of 5 or 30 mg exceeded minimum target therapeutic concentrations and produced a significant reduction in plasma HIV-1 RNA viral load.
Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS. 2015 Jul;10(4):239-45. doi: https://doi.org/10.1097%2FCOH.0000000000000168. PMID: 26049948; PMCID: PMC5638427.
Purpose of review
Long-acting cabotegravir may provide a novel therapeutic option for both the treatment and prevention of HIV-1 infection that does not necessitate adherence to a daily regimen. The present review will highlight the unique formulation properties and pharmacologic attributes of long-acting cabotegravir nanosuspension.
Recent findings
Cabotegravir is a potent integrase strand transfer inhibitor that has been formulated as an oral tablet for daily administration and as a long-acting injectable nanosuspension. Long-acting cabotegravir is readily absorbed following intramuscular and subcutaneous administration and has an elimination half-life of approximately 40 days, allowing for administration on a monthly or less frequent schedule. Repeat-dose pharmacokinetic studies and population pharmacokinetic modeling indicate monthly and bi-monthly dosing achieves clinically relevant plasma concentrations considered effective for HIV maintenance therapy and that quarterly injections are appropriate for investigation as preexposure prophylaxis. Cabotegravir is primarily metabolized by uridine diphosphate glucuronosyltransferase 1A1 and is unlikely to be impacted by the cytochrome P450 metabolic pathway. In vitro and in vivo data suggest cabotegravir has a low propensity to cause, or be subject to, significant drug interactions.
Summary
The pharmacologic profile of long-acting cabotegravir supports its continued development for both treatment and prevention of HIV-1 infection.
Collaborate for development
Consider on a case by case basis, collaborating on developing long acting products with potential significant public health impact, especially for low- and middle-income countries (LMICs), utilising the referred to long-acting technology
Share technical information for match-making assessment
Provide necessary technical information to a potential partner, under confidentiality agreement, to enable preliminary assessment of whether specific medicines of public health importance in LMICs might be compatible with the referred to long-acting technology to achieve a public health benefit
Work with MPP to expand access in LMICs
In the event that a product using the referred to long-acting technology is successfully developed, the technology IP holder(s) will work with the Medicines Patent Pool towards putting in place the most appropriate strategy for timely and affordable access in low and middle-income countries, including through licensing